C. Applications
C. Applications
2. Description of Special Applications
3. Column Chromatography (fractional conversions)
1. Demineralization of Water
One of the most important applications is the demineralization of water, which can be performed in two different ways:
a) Demineralization in two separate columns
1 volume of strongly acidic cation exchanger in the H+-form (e.g. SERDOLIT® Red) and approx. 2 volumes of strongly basic anion exchanger in the OH-form (e.g. SERDOLIT® Blue, which has only about half the capacity of SERDOLIT® Red) are packed into two columns and connected in series. Then the passage of water through the two columns is started. Of the salts dissolved in the water (prevalently calcium hydrogen carbonate) cations are exchanged in the Serdolit Red in a counter-current manner against H+- ions. The region of the column saturated with calcium is indicated as a yellow band. The eluate of the first column contains mainly free carbonic acid and mineral acids. The pH is usually in the region of 2 - 4. In large installations, the carbon dioxide is removed by degassing. Serdolit Blue now takes up the eluted anions and exchanges them against OH− - ions which react to water with the protons coming from the cation exchange column. This way, both OH- and H+ are removed out of the equilibrium and the exchange of the contaminating salt ions is complete. The anion exchanger changes the colour from blue to light yellow.
b) Demineralization in a mixed bed column
For some applications – especially when dealing with not too large volumes – we recommend our mixed bed ion exchangers SERDOLIT® MB, MB-1 and MB-2, which are ready for use (after long storage rinse with distilled water before use).
c) Regeneration of mixed bed ion exchangers
A regeneration of mixed bed ion exchangers is possible but requires at first a physical separation of the resins. It is a rather tedious process and only worth when dealing with larger quantities.
The physical separation can be effected by making use of the different densities of the two resins. The resin mixture is removed from the column and placed into a vessel together with the same volume of water. An aqueous solution of 0.5 kg sodium hydroxide per liter is added until the anion exchanger floats in the caustic and is well separated from the cation exchanger remaining at the bottom of the vessel. The anion exchanger is then decanted and filtered on glass fiber paper, washed with 2n NaOH and with distilled or demineral ized water. The cation exchanger is filtered dry, washed to approximate neutrality and then regenerated with HCI according to table 6. The regenerated resins are put back into the column. They tend to separate again in water, by gravity. To avoid this, only a minimum volume of water is added to the resins so that the water above the top of the resins is 10 % of the total length of the resin bed. Now a gentle stream of air is, passed upwards through the column. After shutting down the air, the water is rapidly drained from the column until nearly no water covers the resin.
2. Description of Special Applications
a) Cleaning of acrylamide and urea solutions
Some preparations of acrylamide or urea may contain free acrylic acid resp. ammonium isocyanate. These impurities can easily be removed by passing the solution through a small column of mixed bed ion exchanger with exhaustion indicator (SERDOLIT®-MB, cat. No. 45500). Be aware that this resin contains about 50 % water which will dilute the acrylamide resp. urea solution.
b) Purification of formamide
5 g SERDOLIT® MB are added to 100 ml of formamide . The mixture is slowly agitated for 1 h at room temperature and finally filtered. After filtration, the purified formamide is stored at -20 °C. Again it should be kept in mind that the resin contains about 50 % water.
Tip: by passing the formamide through a small resin column which had been percolated with some milliliters of formamide to remove the water, better results will be obtained in shorter time.
c) Desalting of proteins
Protein solutions should never come into direct contact with ion exchange resins in the free base or hydrogen form. There will always be unspecific adsorption, precipitation or even denaturation. Dialysis against a mixed bed resin is however possible: the protein solution is filled into a dialysis tubing (e.g. VISKING, cat. No. 44104 or 44110) and suspended in a 10 % suspension of SERDOLIT® MB in a beaker. The resin is slowly agitated. If exhaustion is indicated by colour change, the resin is removed and replaced by fresh material.
d) Removal of SDS
The unbound (surplus) detergent can be removed in the same way as described above for desalting of proteins.
e) Removal of SERVALYT™ carrier ampholytes
Dialysis against mixed bed ion exchangers is an effective and also gentle method (VISKING dialysis tubing, cat. No. 44104 is recommended). As SERVALYT™ reacts positively with ninhydrin, a negative reaction indicates the end of the dialysis.
f) Selective removal of calcium or transition metal ions
CHELITE CHE and/or CHELITE P in place of conventional cation exchangers will selectively remove di- and trivalent transition metal ions from a buffering solution. The chelating resin should first be equilibrated with the buffering solution to be treated in order to avoid exchange of monovalent ions within the resin. Separation and elution of the ions is done by step-wise lowering the pH (see fig. 7 and 8). Alternatively, transition metal ions can be bound to anion exchangers as negatively charged complexes after adding of complex-forming agents.
3. Column Chromatography (fractional conversions)
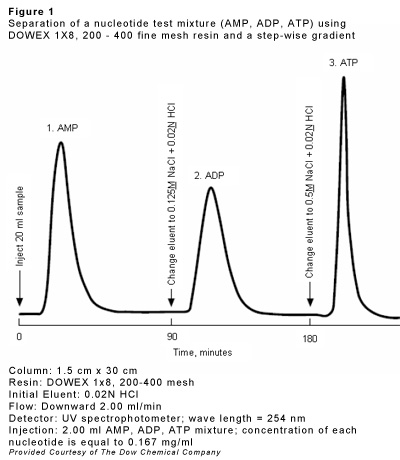
Ion exchange resins can also be used for the chromatography of amino acids, sugar derivatives (borate complexes), nucleotides, antibiotics etc. The column length goes up to 100 times the diameter. Separation is based upon different dissociation constants of these substances. Elution can be performed by a stepwise or continuous change of eluent composition. The latter method is recommended for complex mixtures. The stepwise elution (which is rather a fractional elution rather than a chromatographic process) is particularly advantageous because the individual components will appear in high concentration on defined positions of the eluent.
| Applications | References | |
| a) | Preconcentration and analysis of selected metal ions from various fluids (e.g. blood or beverages) and aqueous solutions | 1 - 28 |
| b) | Separation of organic acids from biological fluids and aqueous solutions | 29 - 31 |
| c) | Separation and analysis of mixtures of amino acids, amino acid derivatives or nucleotides (see fig. 1) | 32 - 39 |
| d) | Separation of other organic compounds | 40 - 49 |
| e) | Use of ion exchangers as catalysts in organic synthesis, e.g. for sugar derivatives, esters, etc. | 50 - 58 |
| f) | Use of ion exchangers for drug delivery purposes | 59 - 61 |
